
Density of fuel oils as function of temperature - Variations in fuel oils density are shown as function of temperatur, together with volume correction factors.
Density of crude oil as function of temperature - Variations in crude oil density are shown as function of temperatur, together with volume correction factors.Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight.Average boiling point - Definition, explanation and examples of calculation of various types of average boiling point of petroleum products and other mixtures of hydrocarbons: VABP, MABP, WABP, CABP and MeABP.Online API to Specific Gravity calculator. API Gravity - API expresses the gravity or density of liquid petroleum products.Alcohols and carboxylic acids - physical data - Molweight, melting and boiling point, density, pKa-values, as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids.Involves velocity, pressure, density and temperature as functions of space and time Fluid Mechanics - The study of fluids - liquids and gases.Material Properties - Material properties for gases, fluids and solids - densities, specific heats, viscosities and more.The difference between MW of gas oil from Eq (2) and (4) is 6.5 %. MeABP can then be found by using correction factors: Then, VABP (volume average boiling point) and the distillation curve slope is calculated: The sample gravity is measured to be of 31.4☊PIįirst, API gravity must be converted to specific gravity: The difference between molecular weight of naphtha from Eq (1) and (3) is 0.2 %.Įxample 2: Molecular weight of a light gas oil from a distillation curveĭ86 used to distille a gas oil sample showes: The results from Example 1 are given as a red X in the figures. See also Average boiling point from gravity and molecular weight and Molecular Weight - Gases and VaporsĬalculate the molecular weight of a naphtha with specific gravtity, S = 0.763 and a MeABP of 292☏ (2) and (4) are used to calculate the molecular weight for a number of specific gravities and boiling points and the results, given with ☌ and ☏, are shown in the figures below The average error is said to be about 7%.Įq. These relationships are valid in the ranges: For heavier oil samples equation (3) and (4) are recommended. T K = MeABP (mean average boiling point), in K (degree Kelvin)Įquation (1) and (2) fail to properly predict properties for hydrocarbons above C 25, with boiling point > 400☌ (750☏). T R = MeABP (mean average boiling point), in °R (degree Rankin) Two of the most used models, recommended by API, are given below: However, there are several models calculating average molecular weight, based on gravity and distillation data. Direct measurement of molecular weight is time demanding and gives imprecise results. But, exact analysis of the composition of complex mixtures of hydrocarbons, such as crude oil or oil distillation fractions, which can consist of several thousand different compounds, is difficult to get.
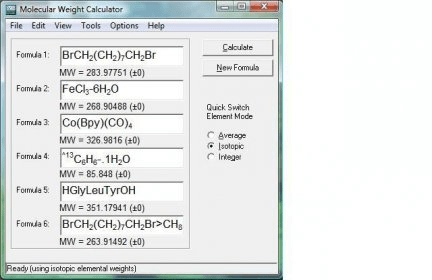
For many purposes in chemical engineering, there is important to know the molecular weight (MW) of the fluids in process streams, and for mixtures of several different compounds an average molecular weight can be useful.


 0 kommentar(er)
0 kommentar(er)
